News
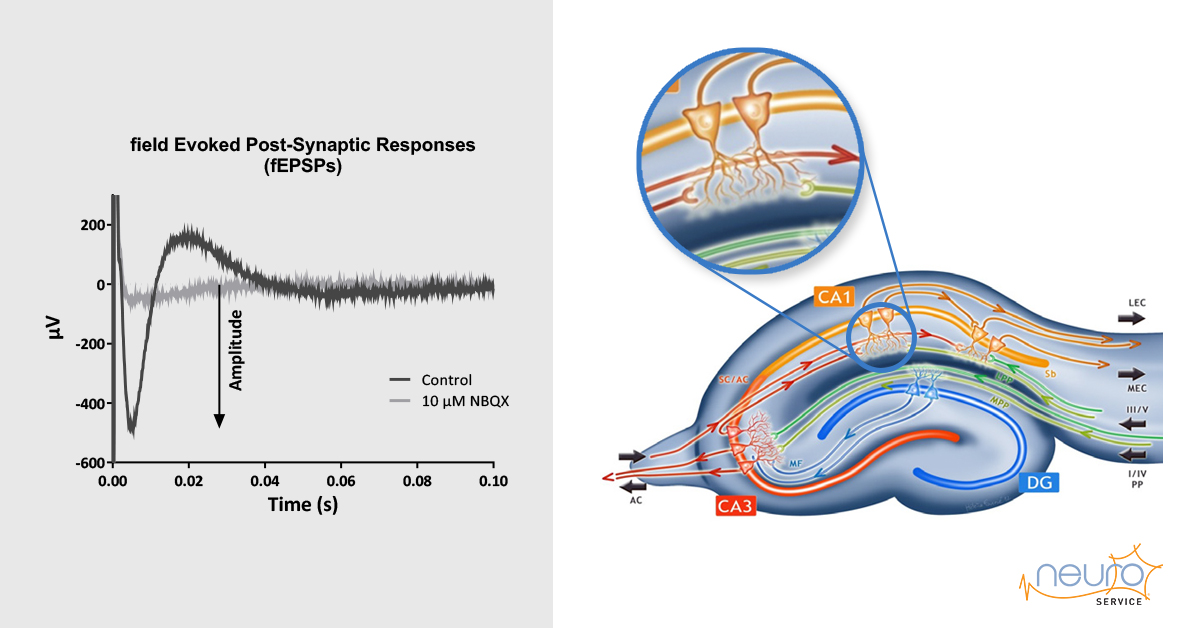
Transgenic Alzheimer’s Disease mice slice electrophysiology
Tg2576 and ARTE10 mice models express behavioral and cognitive deficits characteristic of Alzheimer’s Disease.
NEUROSERVICE documented the strength of several electrophysiological endpoints (synaptic transmission, short-term and long-term plasticity) in the hippocampal CA1 region to examine potential deficits in comparison to WT counterparts.
The aim was to determine the age of the deficit onset and if they worsen as the mice grow old.
ARTE 10 MODEL
The transgenic mouse line ARTE10 was generated by co-integration of two transgenes carrying the K670N/M671L mutated amyloid precursor protein (APPswe) and the M146V mutated presenilin 1 (PS1) both under control of a neuron-specific promoter.
Plaque deposition starts at 3 months in homozygous ARTE10 mice and primarily and predominantly involves the hippocampus, the neocortex, and limbic cortical areas such as the amygdala.By the age of 12 months, ARTE10 mice developed cognitive deficits mainly in episodic memory (Willuweit, 2009). At 10-14 months of age, ARTE10 mice are assumed to display neuronal hyperexcitability at the single cell level but also aberrant circuit synchronization (Siskova, 2016).
Animals: 4 WT ARTE10 mice + 5 CAR ARTE10
The fEPSP amplitude was measured as the difference between the baseline (before stimulation) and the maximal peak amplitude.
The background noise at the level of the individual electrodes was subtracted from the fEPSP amplitude. To be validated, fEPSPs needed to be stable over the baseline period (change < 15%), and higher than 70 μV as well as fully inhibited by 10 μM NBQX. Each slice was considered as an individual sample (n). Data from each individual electrode was averaged for each slice first and next data from each slice was averaged for Wt and CAR mice.
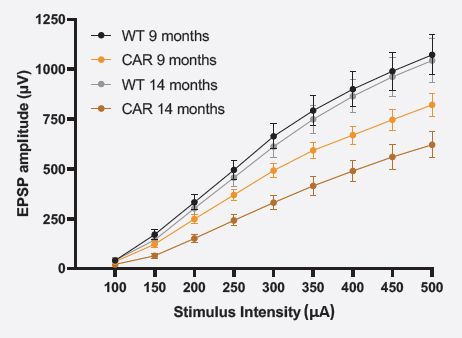
SYNAPTIC TRANSMISSION (I/O)
For the input/output (I/O) curve, raw fEPSP amplitudes were averaged as a function of the stimulation intensity ± SEM.
Synaptic transmission seemed to be impaired in both 9 and 14 months-old CAR mice.
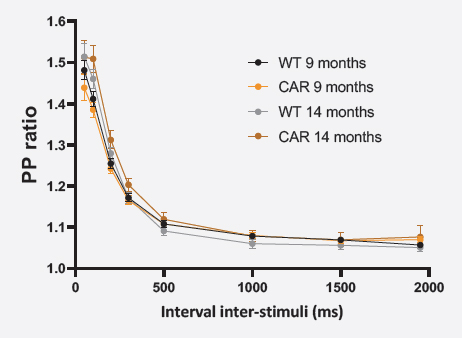
SHORT-TERM PLASTICITY (PPR)
For the paired-pulse protocol, the ratio fEPSP2/fEPSP1 amplitudes were averaged as a function of the inter-stimulus interval ± SEM.
Short-term synaptic plasticity was in the same range in both CAR and WT mice at 9 and 14 months of age.
Download the summary one-page below:
AD mouse model – ARTE10
Tg 2576 MODEL
Animals: 5 WT Tg2576 mice + 5 CAR Tg2576
The fEPSP amplitude was measured as the difference between the baseline (before stimulation) and the maximal peak amplitude.
The background noise at the level of the individual electrodes was subtracted from the fEPSP amplitude.
To be validated, fEPSPs needed to be stable over the baseline period (change < 15%), and higher than 70 µV as well as fully inhibited by 10 µM NBQX.
Each slice was considered as an individual sample (n). Data from each individual electrode was averaged for each slice first and next data from each slice was averaged for Wt and CAR mice.
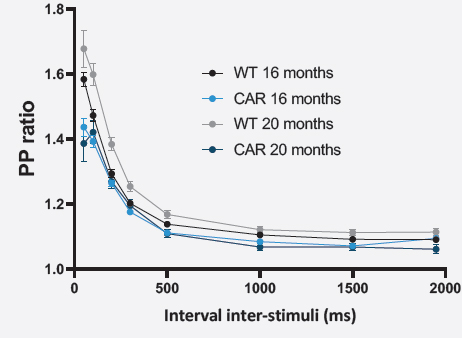
SHORT-TERM PLASTICITY (PPR)
For the paired-pulse protocol, the ratio fEPSP2/fEPSP1 amplitudes were averaged as a function of the inter-stimulus interval ± SEM.
Short-term plasticity appears to be impaired in both 16 and 20 months-old CAR mice
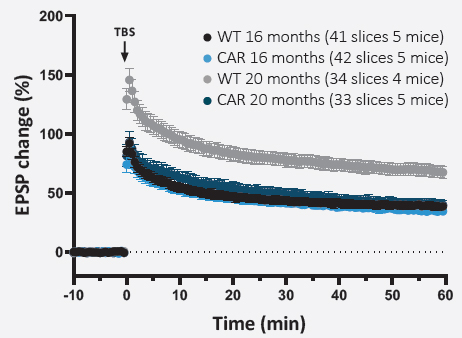
LONG-TERM PLASTICITY (LTP)
For LTP experiments, data were averaged with a 30-second bin.
The percentage change in fEPSPs amplitude was calculated with regards to the baseline. Data were plotted ± SEM versus time.
Scatter plots are presented with mean values at t0’ and t60’ and over the 60 min period after the TBS to assess the potentiation (numerical values of mean potentiation ± SEM are indicated above each dot plot).
Long-term synaptic plasticity appeared to be significantly impaired in 20 months-old CAR mice only.
Download the summary one-page below:

