News
Experimental scientific support to drug discovery programs targeting cation-chloride cotransporters
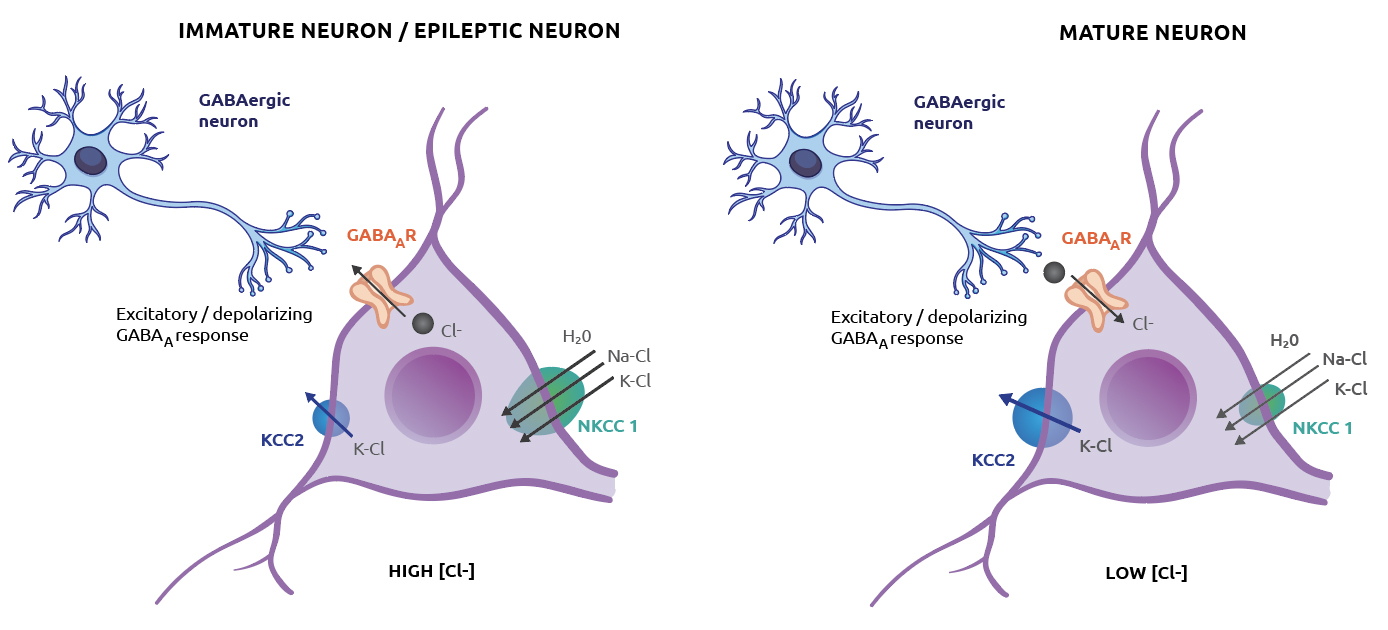
GABA (γ-aminobutyric acid) is the principal inhibitory neurotransmitter of the central nervous system and acts primarily through GABAA receptors which are ligand gated ion channels selectively permeable to the anion, chloride.
In mature neurons, activated GABAA receptors allow for the influx of chloride into the cell thus hyperpolarizing the membrane potential, reducing the probability that neurons will fire an action potential.
Conversely, in early development, GABA is excitatory rather than inhibitory, due to the age-dependent expression of particular cation-chloride cotransporters (CCCs) which are responsible for maintaining the neuronal intracellular concentration of chloride.
Early in development, high NKCC1 expression causes an accumulation of intracellular chloride, whereas in the adult, NKCC1 expression is decreased and the cation-chloride cotransporter KCC2 shows increased levels of expression.
KCC2 extrudes chloride which sets a low intracellular chloride concentration that consequently renders GABAA receptor activation inhibitory.

It is now clear that dysregulation of neuronal chloride homeostasis may underpin some pathologies such as epilepsy, schizophrenia and neuropathic pain as well as developmental disorders such as autism.
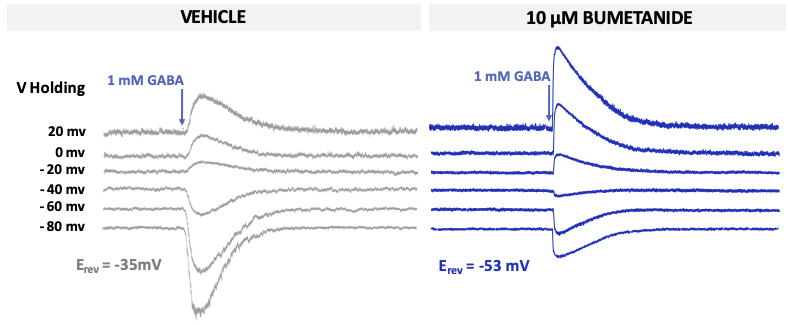
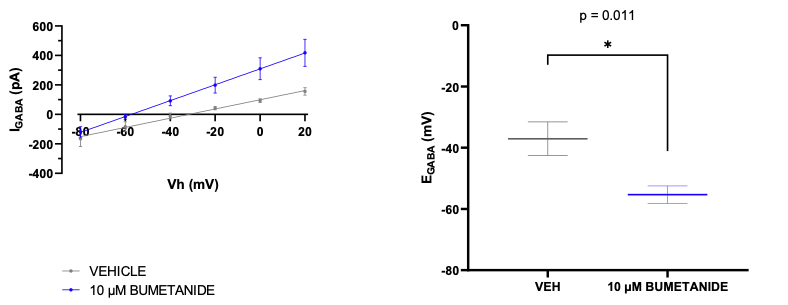
These findings suggest that CCCs could represent promising targets for novel therapeutics. To determine whether GABA is acting in an excitatory or inhibitory manner in neurons, the equilibrium potential for GABA (called EGABA) can be measured using the gramicidin perforated patch-clamp technique.
Molecules which act on CCCs such as bumetanide (a NKCC1 antagonist) alter EGABA as shown in the Figure.
Using the perforated patch-clamp technique, Neuroservice can provide experimental scientific support to drug discovery programs targeting cation-chloride cotransporters.

