News
Cognition studies: your lead compound works in vivo but its mechanism of action (MOA) remains unclear?

Bruno Buisson, founder, general manager & chief scientist at Neuroservice, explains how electrophysiology can illuminate pharmacological compound profile, providing a clearer understanding of mechanism of action, with an in vitro approach for cognition mechanisms on a true neuronal network.
COGNITION STUDIES: INSUFFICIENT INFORMATION ON MECHANISM OF ACTION OF YOUR COMPOUND ?
We meet many customers working on memory, Alzheimer’s Disease or Parkinson’s Disease, who are at advanced stages of development with their molecule with many in vivo data, but who need more information on its target(s) and mechanism of action. Indeed, Regulatory Agencies request more and more information about the understanding of the compounds’ pharmacological activities.
OUR ANSWER: DOCUMENT THE EFFECT OF YOUR MOLECULE ON SYNAPTIC PLASTICITY
Mechanisms of synaptic plasticity are fundamental for basic learning tasks and higher cognitive functions. Indeed, molecular, cellular and physiological investigations have provided compelling evidence that synaptic plasticity is mandatory for learning and memory storage processes, from primary invertebrate organisms to humans. Synaptic plasticity is the ability of synapses to strengthen or weaken their ”weight” over time, in response to neuronal input that encodes signals in frequency modulation.
Two main synaptic plasticity mechanisms have been described: Long-Term Potentiation (LTP) and Long-Term Depression (LTD). Briefly, LTP is triggered by a high-frequency stimulation protocol whereas LTD is induced by a low- frequency stimulation protocol.
In mammals the hippocampus plays a central role in memory tasks (notably declarative memory and spatio-temporal memory). The hippocampus is often used as a “native network” substrate for investigating synaptic plasticity mechanisms either in vitro or in vivo.
Both LTP and LTD depend on NMDA receptor activation and intracellular calcium mobilization. Compounds that are able to modulate NMDA receptor function may therefore impact memory/cognition processes. The role of LTP/LTD in learning/cognitive tasks has been amply illustrated. Animals treated with NMDA receptor antagonists display severely impaired performance in behavioral assays for learning/spatial recognition (Morris water maze, for instance).
Pharmacological or genetic manipulations that interfere with LTP in vitro often affect learning or memory in vivo (ex: Scopolamine or MK-801 treated animals). In addition, LTP is impaired in genetic models of neurodegenerative diseases (Alzheimer’s Disease, Huntington’s Disease…) and in aged animals.
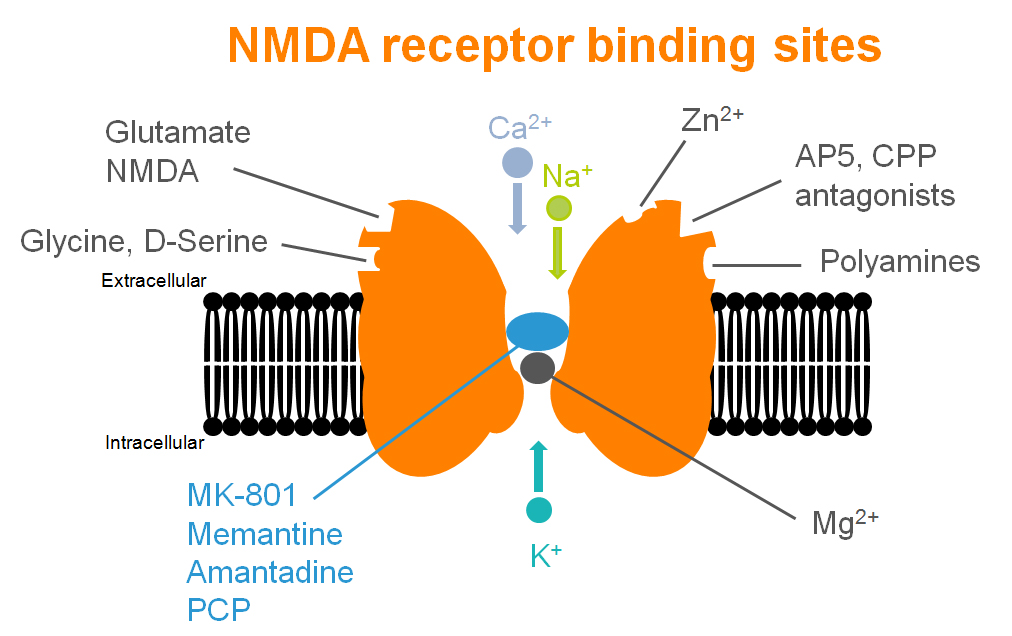
Even if the NMDA receptor has been illustrated as a central key in many synaptic plasticity mechanisms and cognitive processes, many others receptors – and associated pathways – could be involved.
HOW TO INVESTIGATE SYNAPTIC PLASTICITY WITH OUR ELECTROPHYSIOLOGICAL RECORDING TECHNIQUES?
One of our techniques, the Multi-Electrode Array (MEA) technology, enables parallel and multi-site extracellular recordings within a single brain slice and provides an exceptional macroscopic view of neuronal networks function.
MEA recordings from rodent brain slices provide a ready means to evaluate the effect of compounds on synaptic plasticity mechanisms that are crucial for memory and cognition processes.
A compound’s target can be investigated much more easily with MEA recordings vs. in vivo recordings thanks to the ease with which pharmacological tools can be used. Importantly, compounds are evaluated on a true neuronal network (with native receptors), conditions very close to the in vivo situation.
The MEA technique is ideal to bridge the gap between cellular and in vivo assays, to confirm compound activities and/or to investigate their mechanism(s) of action.
- Evoked-responses (excitatory post-synaptic potentials, EPSP) can be recorded in brain slices with Multi-Electrode Arrays (MEA).
- Long-Term Potentiation (LTP) or Long-Term Depression (LTD) can be recorded in the CA1 region of hippocampal slices, and these mechanisms are known to be NMDA-receptor dependent.
Keep in mind that excitatory synaptic transmission (mediated by glutamate) is continuously balanced by mechanisms of inhibitory synaptic transmission (mediated by GABA).
EXAMPLES OF MEA-BASED EXPERIMENTAL INVESTIGATIONS OF SYNAPTIC PLASTICITY MECHANISMS
Determination of LTD/Neutral/LTP crossover point
Stimulation trains over a wide range of frequencies (from 1 to 200 Hz) provide a means to determine the LTD/LTP “crossover point”.
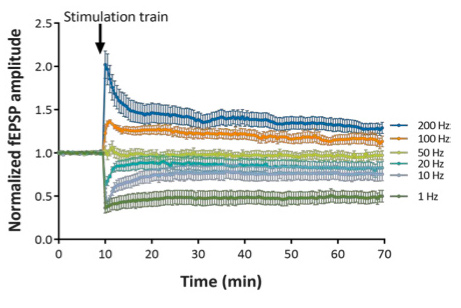
At 100 Hz and 200 Hz, the stimulations train induces Long-Term Potentiation (LTP) of evoked responses.
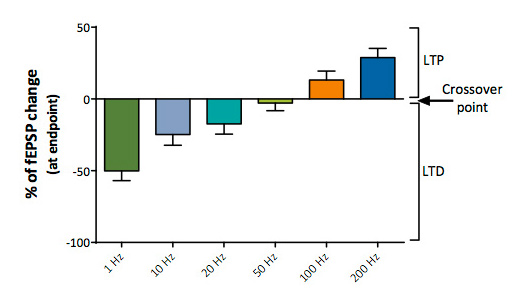
Determination of the whole LTD/LTP profiles in control conditions and in the presence of a compound provides a unique opportunity to investigate the pharmacological properties of putative cognitive enhancers over a wide range of frequence stimulation and to document any unbalancement between LTD/LTP triggered by a molecule.
Evaluation of NMDA non-competitive antagonists (open-channel blocker) on LTP
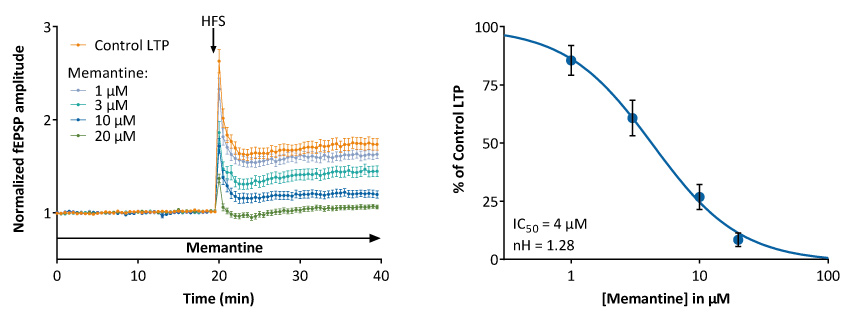
Illustrated is the dose-dependent inhibition of LTP by the non-competitive NMDA receptor antagonist, Memantine, in the CA1 region of rat hippocampal slices.
It is of value to stress out that Memantine does not only interfere with NMDA receptors, but also nicotinic receptors and other ion channels, and that its in vivo pro-cognitive effects may results from pleiotropic target effects.
Evaluation of a NMDA antagonists on LTD
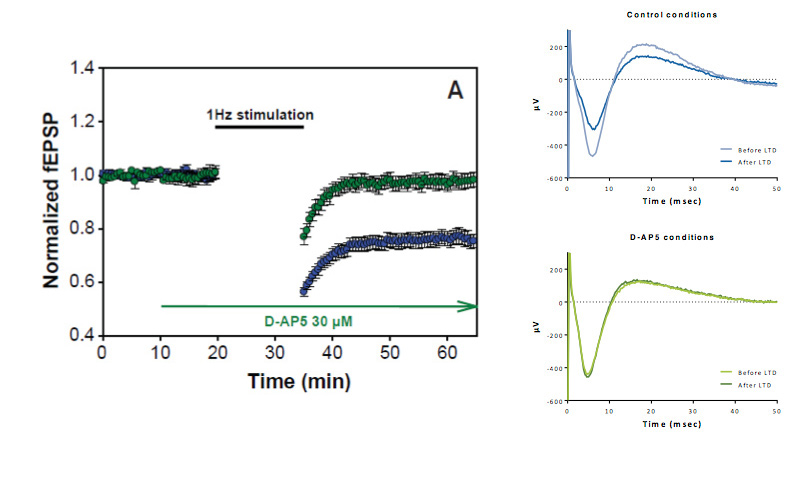
A low frequency stimulation-induced LTD in the CA1 region of rat hippocampal slices. LTD induction is completely blocked by 30 μM of the NMDA receptor antagonist D-AP5.
Investigation of allosteric interactions at NMDA receptors
Complex allosteric interactions at NMDA receptors can be documented as well by LTP recordings. As illustrated below LTP can be partially inhibited by a mid-concentration of MK-801. This partial block can be prevented when the slice is co-incubated by D-Serine, which is known as a co-agonist or allosteric modulator of NMDA receptors.
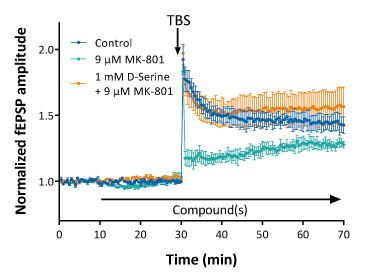
LTP induced by Theta-Burst Stimulation (TBS) is largely decreased by MK-801 (with an approximate IC50 of 9 μM). Pre-exposure with 1 mM D-Serine prevents the MK-801-induced LTP disruption.
Such experiments illustrate the possibility to investigate drug-drug interaction mechanisms at the receptor level in true physiological conditions.
Evaluation of NMDA antagonists on NMDA-mediated EPSP
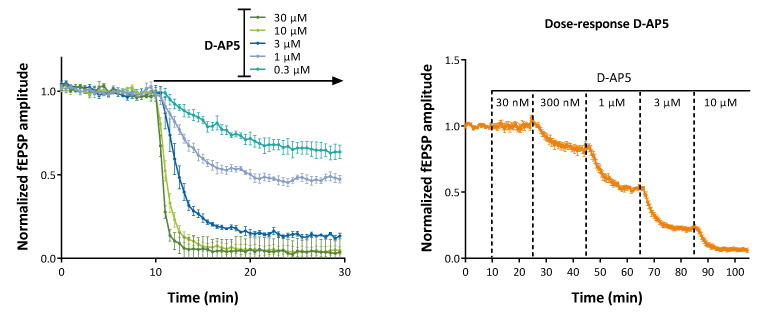
Under certain experimental conditions (low magnesium + NBQX) , “pure” NMDA-mediated EPSPs can be recorded in the CA1 region of hippocampal slices.
“Pure” NMDA-mediated EPSPs provide a ready means to evaluate the actions of NMDA antagonists or NMDA modulators (PAMs/NAMs)
Concentration ranges can be evaluated on independent slices (above left) or sequentially on the same hippocampal slice (above right).
We are also assayable to document synaptic plasticity deficits in slices from Transgenic (Tg) Animals:
– Tg2576 model (Alzheimer’s Disease mouse model)
– R6/2 model (Huntington’s Disease mouse model)
Compounds could be evaluated on “synaptic deficits” in slices prepared from Transgenic Animals.

